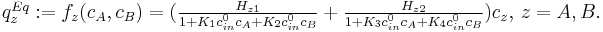| Line 27: | Line 27: | ||
<math> |
<math> |
||
\left\{ |
\left\{ |
||
| − | \begin{array}{ll} |
||
\frac{\partial c_z}{\partial t}+\frac{1-\epsilon}{\epsilon} \frac{\partial q_z}{\partial t} |
\frac{\partial c_z}{\partial t}+\frac{1-\epsilon}{\epsilon} \frac{\partial q_z}{\partial t} |
||
= - \frac{\partial c_z}{\partial x} |
= - \frac{\partial c_z}{\partial x} |
||
| − | +\frac 1 {\bf {Pe}} \frac{\partial ^2 c_z}{\partial x^2} , |
+ | +\frac 1 {\bf {Pe}} \frac{\partial ^2 c_z}{\partial x^2} , |
\frac{\partial q_z}{\partial t} =\frac {L}{u} Km_z ( q_z^{Eq} - q_z), |
\frac{\partial q_z}{\partial t} =\frac {L}{u} Km_z ( q_z^{Eq} - q_z), |
||
\end{array} |
\end{array} |
||
| − | + | t>0,0< x<1, |
|
</math> |
</math> |
||
where <math>c_z,q_z</math> are concentrations of component <math>z</math> in the liquid |
where <math>c_z,q_z</math> are concentrations of component <math>z</math> in the liquid |
||
| − | and solid phase, and < |
+ | and solid phase, and <math>q_z^{Eq}</math> is the adsorption equilibrium concentration defined as |
<math> |
<math> |
||
\begin{array}{ll} |
\begin{array}{ll} |
||
Revision as of 11:24, 20 November 2012
Description of physical model
Preparative liquid chromatography as a crucial separation and purification tool has been widely employed in food, fine chemical and pharmaceutical industries. Chromatographic separation at industry scale can be operated either discontinuously or in a continuous mode. The continuous case will be addressed in the benchmark SMB, and here we focus on the discontinuous mode -- batch chromatography.
The principle of batch elution chromatography for the binary separation is shown schematically in Fig.1 below. During the injection period
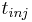 , a mixture consisting of A and B is injected at the inlet of the column packed with a suitable stationary phase.
With the help of the mobile phase, the feed mixture then flows through the column. Since the solutes to be separated exhibit different
adsorption affinities to the stationary phase, they move at different velocities in the column, and thus separate from each other
when exiting the column. At the column outlet, component A is collected between cutting points
, a mixture consisting of A and B is injected at the inlet of the column packed with a suitable stationary phase.
With the help of the mobile phase, the feed mixture then flows through the column. Since the solutes to be separated exhibit different
adsorption affinities to the stationary phase, they move at different velocities in the column, and thus separate from each other
when exiting the column. At the column outlet, component A is collected between cutting points 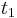 and
and 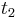 ,
and component B is collected between
,
and component B is collected between 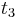 and
and 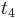 . Here the positions of
. Here the positions of 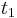 and
and 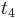 are determined by a minimum concentration threshold that the detector can resolve. The positions of
are determined by a minimum concentration threshold that the detector can resolve. The positions of 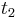 and
and 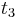 are determined by the purity specifications imposed on the products. After the cycle period
are determined by the purity specifications imposed on the products. After the cycle period 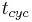 , the injection is repeated.
The feed flow-rate
, the injection is repeated.
The feed flow-rate  and injection period
and injection period 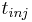 are often considered as the operating variables.
By properly choosing them, the process can achieve the desired performance criterion, such as production rate, while respecting
the product specifications (e.g., purity, recovery yield).
are often considered as the operating variables.
By properly choosing them, the process can achieve the desired performance criterion, such as production rate, while respecting
the product specifications (e.g., purity, recovery yield).
The batch chromatography can be described as the following convection-diffusion system,
Failed to parse (syntax error): \left\{ \frac{\partial c_z}{\partial t}+\frac{1-\epsilon}{\epsilon} \frac{\partial q_z}{\partial t} = - \frac{\partial c_z}{\partial x} +\frac 1 {\bf {Pe}} \frac{\partial ^2 c_z}{\partial x^2} , \frac{\partial q_z}{\partial t} =\frac {L}{u} Km_z ( q_z^{Eq} - q_z), \end{array} t>0,0< x<1,
where 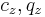 are concentrations of component
are concentrations of component 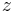 in the liquid
and solid phase, and
in the liquid
and solid phase, and 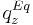 is the adsorption equilibrium concentration defined as
is the adsorption equilibrium concentration defined as